Sialic acid
 Sialic acid |
Sialic acid is a generic term for the N-or O-substituted derivatives of neuraminic acid, a monosaccharide with a nine-carbon backbone. It is also the name for the most common member of this group, N-acetylneuraminic acid (Neu5Ac or NANA). Sialic acids are found widely distributed in animal tissues and to a lesser extent in other species ranging from plants and fungi to yeasts and bacteria, mostly in glycoproteins and gangliosides. The amino group generally bears either an acetyl or glycolyl group but other modifications have been described. The hydroxyl substituents may vary considerably: acetyl, lactyl, methyl, sulfate, and phosphate groups have been found. The term "sialic acid" (from the Greek for saliva, σ?αλον/sialon) was first introduced by Swedish biochemist Gunnar Blix in 1952. |
| Contents |
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
Product: N-Acetylneuraminic acid |
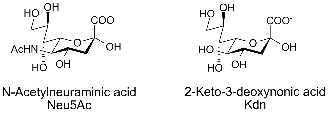 The numbering of the sialic acid structure begins at the carboxylate carbon and continues around the chain. The configuration which places the carboxylate in the axial position is the alpha-anomer. |
|
|
||||
|
Form |
crystalline |
|
Hazard Code |
Xi |
|
mp |
184-186 °C (dec.)(lit.) |
|
Risk Phrases |
36/37/38 |
|
Specific Rotation |
-32 o (C=2,water) |
|
Safety Phrases |
22-24/25-36-26 |
|
Index of Refraction |
-32 ° (C=1, H2O) |
|
WGK Germany |
3 |
|
Solubility |
50 g/L H2O (20°C) |
|
F |
3-10-23 |
|
Sensitivity |
Air Sensitive |
|
Hazard Note |
Irritant |
|
Storage |
?20°C |
|
HS Code |
29329970 |
|
|
|
|
|
|
|
It plays a very important role in the production and development of brain and nervous system. Researches show that decreasing ganglioside has something to do with early malnutrition and learning disability. A proper complement of sialic acid is good either to behavior learning improvement or early brain development. |
Seebio Biotech, Inc. provides N-Acetylneuraminic acid as follows.
| Code | Product | CASN | Grade |
| DLK0011A | N-Acetylneuraminic acid (Sialic acid) | 131-48-6 | ≥99.0% |
11-502. Lane 299, Bisheng Rd.
Zhangjiang High Tech Park
Shanghai 201204, P.R.China
Tel: 86-21-50272975, 76, 77, 78, 79, 81; Ext:6513, 6515, 6516, 6520, 6521, 6522
For customers' complaint or suggestion: market5@seebio.cn
Web: www.seebio.cn
Email: fyland@seebio.cn; service@seebio.cn
MSN:seebio8@hotmail.com
Skype:seebiocn
 |
 |
 |
| 官网:www.cxbio.com | 微信服务号:iseebio | 微博:seebiobiotech |
 |
 |
 |
| 商城:mall.seebio.cn | 微信订阅号:seebiotech | 泉养堂:www.canmedo.com |
相关资讯
- Sigma Supelco 离子液体气相毛细管色谱柱
- 如何使用抗-PEG琼脂糖亲和纯化mPEG-BSA
- 科学家有望利用鼻细胞成功治疗人类脊髓损伤
- α-酮戊二酸|α-Ketoglutaric acid|328-50-7|西宝生物
- Cancer Discovery:KRAS诱导线粒体自噬来促进胰腺癌发展
- 药用辅料 - 卡波姆(carpol)
- 美国多家诊断公司获允提供院外诊断服务
- Miscellaneous 其它低聚糖 - Elicityl细菌发酵生产低聚葡聚糖产品 (9)
- 纳斯达克生物科技股的泡沫来了吗?
- Cell:重磅!揭示免疫检查点抑制剂攻击癌症机制
新进产品
同类文章排行
- 胆碱酯酶检测原料一览
- 猴痘病毒检测原料(涵盖诊断酶、抗原、抗体),西宝生物助力猴痘检测
- 西宝生物CHO细胞、HEK293细胞、 Vero细胞培养基产品
- 人和动物免疫球蛋白IgG全新上市
- 如何选择合适的表面活性剂?
- 尿液分析试纸检测原理及产品推荐
- 总胆红素/直接胆红素检测一站式解决方案
- 分子诊断用酶原料一站式服务尽在西宝生物
- 体外诊断中交联剂的用途与选择
- 优异的化妆品级水溶性β-1,3-葡聚糖
资讯文章
您的浏览历史